Do advanced age and allergies contribute to the reactivation of dormant herpes viruses?
What is this research project about?
Older people may suffer from weakened immune responses
What is this research project about?
Elderly individuals often show impaired adaptive immune responses towards vaccines. One underlying mechanism, called immune senescence, is a narrowing of the repertoire of immune receptors on white blood cells (B cell and T cell receptors), which reduces the flexibility of the immune system to respond to pathogens. This immune senescence may underlie secondary outbreaks of latent Varicella Zoster (VZV) infections in the elderly, which happens at a relatively high frequency of 1.5 to 5 in 1000. Interestingly, this frequency does not seem to be reduced in individuals that had been vaccinated against VZV during childhood.
What’s the current status?
Patients with atopic diseases (“allergic” diseases) are often more susceptible to viral infections. For example, a subgroup of patients suffering from atopic dermatitis (AD) shows an increased susceptibility towards herpes simplex virus (HSV-1). This disease can result in recurrent, severe and disseminated infections (“eczema herpeticum”) bearing the risk of dangerous herpes encephalitis. To address this problem, two VZV vaccines are available for adults at the moment. While a live vaccine with an attenuated strain leads to protection in about 51 % of cases, a new vaccine based on a recombinant protein in combination with two adjuvants works significantly better (>90 % protection). However, a subgroup of subjects who did not respond in clinical studies remains and this group has not been characterized to date.
How do we get there?
The project is based on the collection of already existing biosamples from patients with atopic dermatitis. In addition, we are currently collecting biosamples from the Zoster cohort and the HSV cohort. The samples of the RESIST study with the general population of Hannover serve as controls. Further, samples from elderly persons before and after VZV vaccination will be investigated. Here, the overall aim is to investigate anti-viral T cell responses in detail.
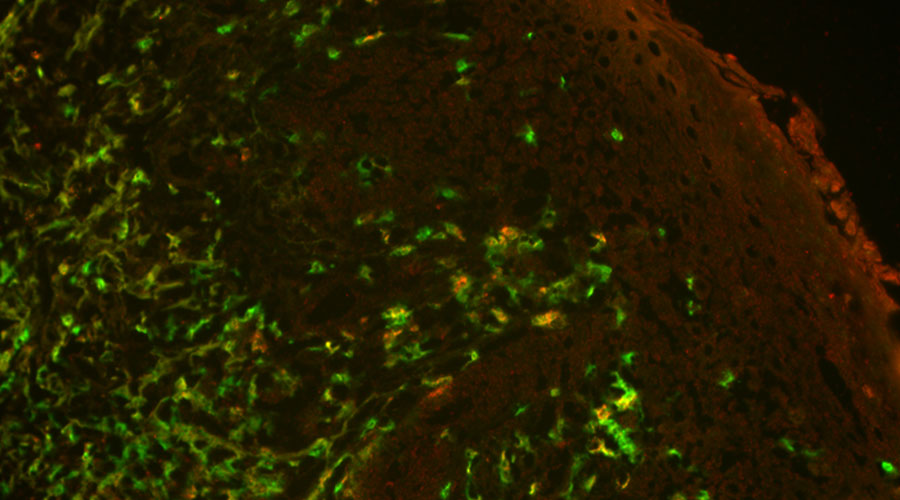
With our long-standing experience regarding innate and adaptive immunity and the inflammatory processes in patients suffering from AD, we are in a unique position to investigate the immune response in susceptible individuals. In previous in vitro studies involving affected patients, we found increased frequencies of T cells directed against HSV-1 that showed a probably ineffective immune response (Traidl et al. 2018). These findings suggested that the atopy-biased inflammatory milieu in these patients leads to the generation of virus-specific T cells that harbor a sub-optimal immune response against viruses. Applying our established set of methods to longitudinally characterize the dynamics of T cell responses, we aim to identify and will perform a deep phenotypical and functional characterization of pathogenic αβ as well as γδ T cells.