How is immune senescence characterised? Does age have a general or specific effect on immune responses against viruses?
Prof. Osterhaus | Prof. Rimmelzwaan
What is this research project about?
It is known that during aging, the functionality of the immune system declines. As a result, elderly persons are more prone to develop cancers and are more susceptible to infection with viral, bacterial and parasitic pathogens. Examples are the increased susceptibility to infections with influenza viruses and Respiratory Syncytial Virus (RSV) that can cause severe respiratory disease. Another consequence of the reduced function of the immune system is that elderly persons do not respond to vaccination as well as younger individuals. Therefore, the influenza vaccine effectiveness in the elderly is suboptimal. A better understanding of the mechanisms of reduced responsiveness to infection or vaccination and immune control over persistent infections (also known as immunosenescence), like those caused by Varicella Zoster Virus (VZV) and of the defects of the elements that compose the immune system may aid the development of better intervention strategies and improved vaccines. Here our main interest is immunity to virus infections in individuals with different levels of immune-senescence.
What’s the current status?
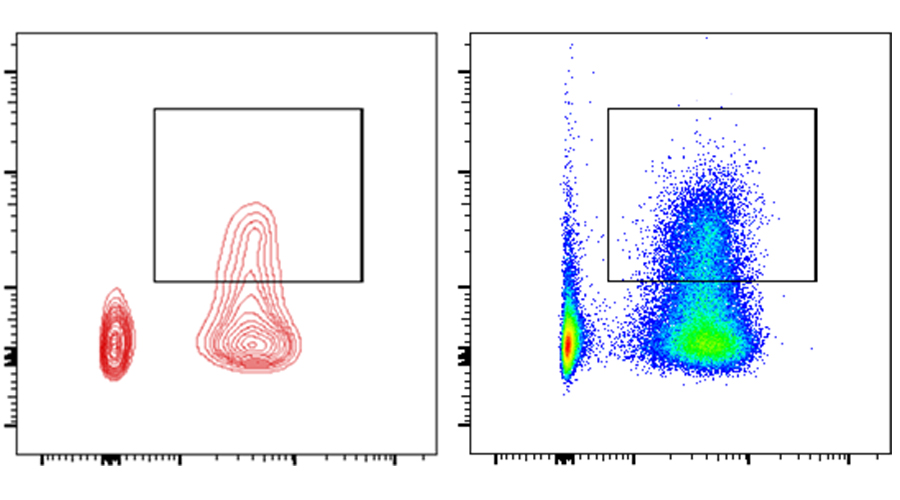
The immune system is composed of various components and cells. In addition to components that respond to virus infection and vaccination in a non-specific way, cells of the adaptive immune system recognize their targets in a virus-specific way. The adaptive immune system against virus infections consists of virus specific B and T lymphocytes. B cells produce antibodies that can e.g. neutralize virus and prevent infection whereas T cells either regulate specific responses of B cells and T cells with other effector mechanisms. Upon stimulation T cells proliferate and acquire effector functions, that include production of cytokines and/or exertion of lytic and other control activities against virus-infected cells. Also memory cells are formed which lead to recall responses upon repeated encounters with the same or closely reated viruses. Immunosenescence may be related to failure to exert the effector functions of (memory) T cells properly. Identification of these defects as biomarkers for immunosenescence during aging will provide a lead towards improved anti-viral T cell function in the elderly.
How do we get there?
Using peripheral blood mononuclear cells obtained from cohorts of study subjects of various ages (young and older adults) and study subjects with or without episodes of VZV, T cell immunity and the presence of antibodies to RSV, influenza virus and VZV will be assessed. We will be using the Zoster cohort and age-matched control subjects from the RESIST cohort with citizens from Hannover to study immunity to other (respiratory) viruses. Specifically, we will study the functional properties of the virus specific T cells by determining their frequencies, their capacity to proliferate, produce cytokines and exert lytic activity. Comparing the T cell function of the respective study groups and virus specificities will teach us whether signs of immune-senescence are virus specific or more generic, which might shed light on the overall increased susceptibility to virus infection and reactivation of VZV due to reduced immune control.