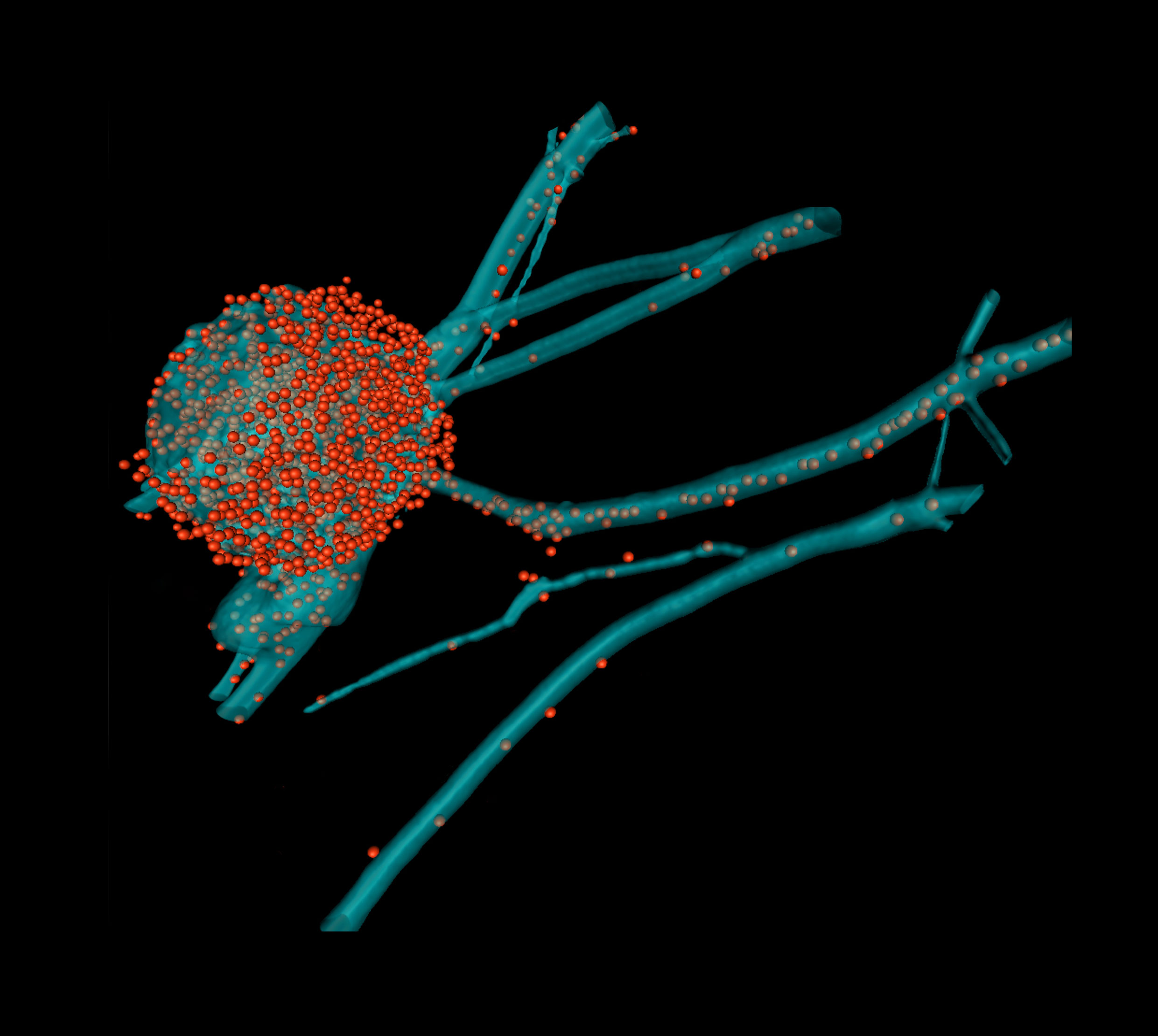
What new therapeutic options open up, from research into the assembly process of herpesviruses
What is this research project about?
While a healthy immune system is able to control herpesviruses, primary and recurrent infections can cause severe disease; particularly very early in life and in the elderly as well as in individuals with increased susceptibility, either due to genetic factors or to immune suppression, e.g., after organ transplantation or for those living with HIV/AIDS. Due to their ubiquitous prevalence, the disease burden of herpesviruses is high in industrialized and developing countries. Diseases range from stigmatizing skin lesions to unbearable pain, life-threatening encephalitis and cancers. The human herpesviruses responsible for the most serious and even life-threatening complications are human cytomegalovirus (HCMV), Herpes Simplex virus (HSV-1 and HSV-2), Varicella Zoster Virus (VZV), and Kaposi Sarcoma Herpesvirus (KSHV).
What’s the current status?
Only few licensed drugs are in clinical use to treat herpesvirus infections, and these target mainly enzymes required for viral DNA replication. Furthermore, these drugs have severe side effects, and resistant viral strains emerge upon prolonged treatment. The recently approved Letermovir that inhibits the HCMV terminase, may represent the first drug to expand our scope of antiviral targets. However, first resistant HCMV mutants have already been described indicating that combination therapies – as used successfully against HIV and HCV – are required. To this end, our research aims at the identification and characterization of unique protein-protein interactions that are essential for virion assembly and therefore potentially represent novel druggable targets. This strategy will pave the way for advanced combination therapies with reduced side effects, and a much lower risk of the emergence of drug-resistant viruses in patients.
How do we get there?
We will focus on elucidating the underlying molecular principles how evolutionary conserved protein complexes function in herpesvirus assembly, and unite our unique expertise on crucial steps of herpesviral assembly (in collaboration with Thomas Schulz and Abel Viejo Borbolla). Combined, we have profound experience on sophisticated protein expression strategies, state-of-the-art structural biology (including X-ray crystallography, cryo electron microscopy, and electron cryo tomography), protein biochemistry (e.g. label-free interaction analysis, co-immuno-precipitation, in vitro capsid assembly and tegumentation), herpesvirus genetics (BAC mutagenesis), immunoelectron microscopy of infected cells and tissues, and life cell imaging using strains with fluorescently tagged capsids and tegument. With our broad expertise, we will uncover evolutionarily conserved mechanisms as well as steps that diverge in virus assembly among different herpesviruses.