Which mechanisms lead to severe disease progression after herpes infections in humans with atopic dermatitis?
What is this research project about?
The human alpha herpesviruses herpes simplex virus type 1 (HSV-1), HSV-2 and Varicella Zoster Virus (VZV) cause diseases ranging from painful and stigmatising facial, oral and genital lesions to life-threating encephalitis, meningitis, and disseminating infections. Primary and recurrent infections cause significant morbidity and even mortality particularly in individuals with increased susceptibility, either due to genetic factors or to immune suppression, as well as very early in life and in the elderly. HSV and VZV are the most common infections of the human nervous system, affecting mostly the peripheral nervous system but also infecting the brain. The long-term post-herpetic neuralgia impairs the quality of life for several months to years after an active zoster episode (shingles). Atopic, so-called “allergic”, skin inflammation leads to vulnerability of the skin and increased susceptibility to viral infections can be observed. Disseminating spread of HSV in the skin (eczema herpeticum, EH) can be life threatening and is a common cause for hospitalization of a severely affected subgroup of patients with atopic dermatitis (AD).
What’s the current status?
While several effective drugs such as acyclovir which block viral replication are available to treat infection with alpha herpesviruses, they often can only be applied too late after a full outbreak of clinical symptoms. However, levels in the brain are often too low to be sufficiently effective and patients with disseminated HSV skin infections are at risk for severe complications including herpes encephalitis.
In this project, we want to elucidate why a subgroup of patients suffers particularly from increased susceptibility to HSV and VZV infections, in order to develop new concepts and novel therapeutic strategies. In earlier studies, we generated primary human skin cells (keratinocytes) from hair follicles of patients who suffered from AD, and who had a history of EH (AD/EH). We compared the HSV-1 infection rates in cells from AD/EH patients with those from AD patients without previous EH and to healthy controls. The data clearly indicate that keratinocytes from the EH patients were most susceptible to HSV-1 infection. In former studies we saw that the skin inflammation in AD contributes to a weakened skin barrier function (Seltmann et al. 2015), and to an increased susceptibility to viruses (Traidl et al. 2018). To identify genetic risk factors, we collect patient samples (to date: more than 1,000 patients with chronic cutaneous inflammatory diseases including 500 AD patients), and we perform genetic analyses of patients suffering from AD and with a history of EH.
How do we get there?
We are in a unique position to identify novel primary immune deficiency factors increasing the susceptibility to alpha herpesvirus infections and leading to adverse disease progression. We combine complementary expertise on skin diseases, immunology, clinical neurology, virology, cell biology, and in particular, the combined genetic contribution of the host and the pathogens involved.
Our experience in cell culture systems to study the most relevant cell types for alpha herpesvirus infection; namely, keratinocytes, fibroblasts, immune cells and neurons will help us to build up experimental models to investigate different factors leading to enhanced susceptibility to viruses. We will determine whether the candidate factors identified through the patient cohort induce increased susceptibility to HSV-1 and -2, VZV, smallpox vaccination (MVA), or rhinovirus (RSV).
Towards this end, we develop a murine skin infection model to study the dynamics of HSV-1 spread within the skin, and from the skin to the nervous system. In this system, we will be able to manipulate potential host susceptibility factors, and to study their impact on the progression of viral skin infection. In addition, samples are currently being collected for the HSV cohort.
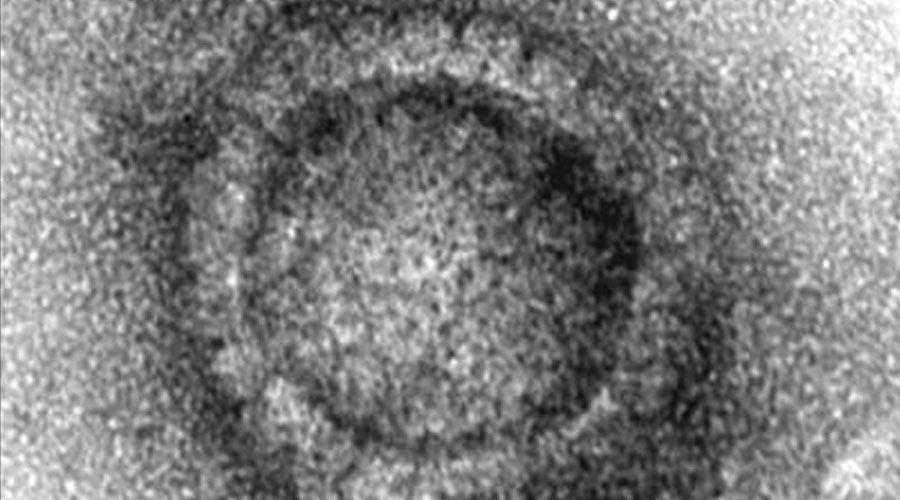
Electron microscope image of a capsid of the herpes simplex virus