Publikationen 2025
Distinct Inflammatory Imprint in Non-Cirrhotic and Cirrhotic Patients Before and After Direct-Acting Antiviral Therapy M. Witte, C. Oltmanns, J. Tauwaldt, H. Schmaus, J. Mischke, G. Grabert, et al. Clin Mol Hepatol 2025 Accession Number: 40462644 DOI: 10.3350/cmh.2025.0292
Publications 2024
Unraveling the dynamics of hepatitis C virus adaptive mutations and their impact on antiviral responses in primary human hepatocytes. Frericks N, Brown RJP, Reinecke BM, Herrmann M, Brüggemann Y, Todt D, Miskey C, Vondran FWR, Steinmann E, Pietschmann T, Sheldon J. J Virol. 2024 Feb 6:e0192123. doi: 10.1128/jvi.01921-23. Online ahead of print. PMID: 38319104
Elevation of S2-bound α1-acid glycoprotein is associated with chronic hepatitis C virus infection and hepatocellular carcinoma. Oltmanns, C., B. Bremer, L. Kusche, P. Stål, R. Zenlander, J. Tauwaldt, I. Rydén, P. Påhlsson, M. Cornberg, and H. Wedemeyer. 2024. J Viral Hepat.
Publications 2023
Reverse inflammaging: Long-term effects of HCV cure on biological age. Oltmanns C, Liu Z, Mischke J, Tauwaldt J, Mekonnen YA, Urbanek-Quaing M, Debarry J, Maasoumy B, Wedemeyer H, Kraft ARM, Xu CJ, Cornberg M. J Hepatol. 2023 Jan;78(1):90-98.
Proteomics reveals a global phenotypic shift of NK cells in HCV patients treated with direct-acting antivirals. Bi W, Kraft A, Engelskircher S, Mischke J, Witte M, Klawonn F, van Ham M, Cornberg M, Wedemeyer H, Hengst J, Jänsch L. Eur J Immunol. 2023 Jul 29:e2250291.
Structural insights into hepatitis C virus neutralization. Ströh LJ, Krey T. Curr Opin Virol. 2023 Mar 29;60:101316.
Immunological scars after cure of hepatitis C virus infection: Long-HepC? Cornberg M, Mischke J, Kraft AR, Wedemeyer H. Curr Opin Immunol. 2023 Apr 10;82:102324.
Controlled Attenuation Parameter Is Associated with a Distinct Systemic Inflammatory Milieu after Clearance of HCV Infection. Du Y, Khera T, Liu Z, Tudrujek-Zdunek M, Dworzanska A, Cornberg M, Xu CJ, Tomasiewicz K, Wedemeyer H. Biomedicines. 2023 May 25;11(6):1529. doi: 10.3390/biomedicines11061529. PMID: 37371624; PMCID: PMC10295384.
Publications 2022
Entwicklungsansätze für Impfstoffe gegen Hepatitis-C-Virus-Infektionen [Development approaches for vaccines against hepatitis C virus infections]. Bankwitz D, Krey T, Pietschmann T. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022 Feb;65(2):183-191. German. doi: 10.1007/s00103-021-03477-9. Epub 2022 Jan 11. PMID: 35015104; PMCID: PMC8749110.
A Hepatitis C virus genotype 1b post-transplant isolate with high replication efficiency in cell culture and its adaptation to infectious virus production in vitro and in vivo. Heuss C, Rothhaar P, Burm R, Lee JY, Ralfs P, Haselmann U, Ströh LJ, Colasanti O, Tran CS, Schäfer N, Schnitzler P, Merle U, Bartenschlager R, Patel AH, Graw F, Krey T, Laketa V, Meuleman P, Lohmann V. PLoS Pathog.
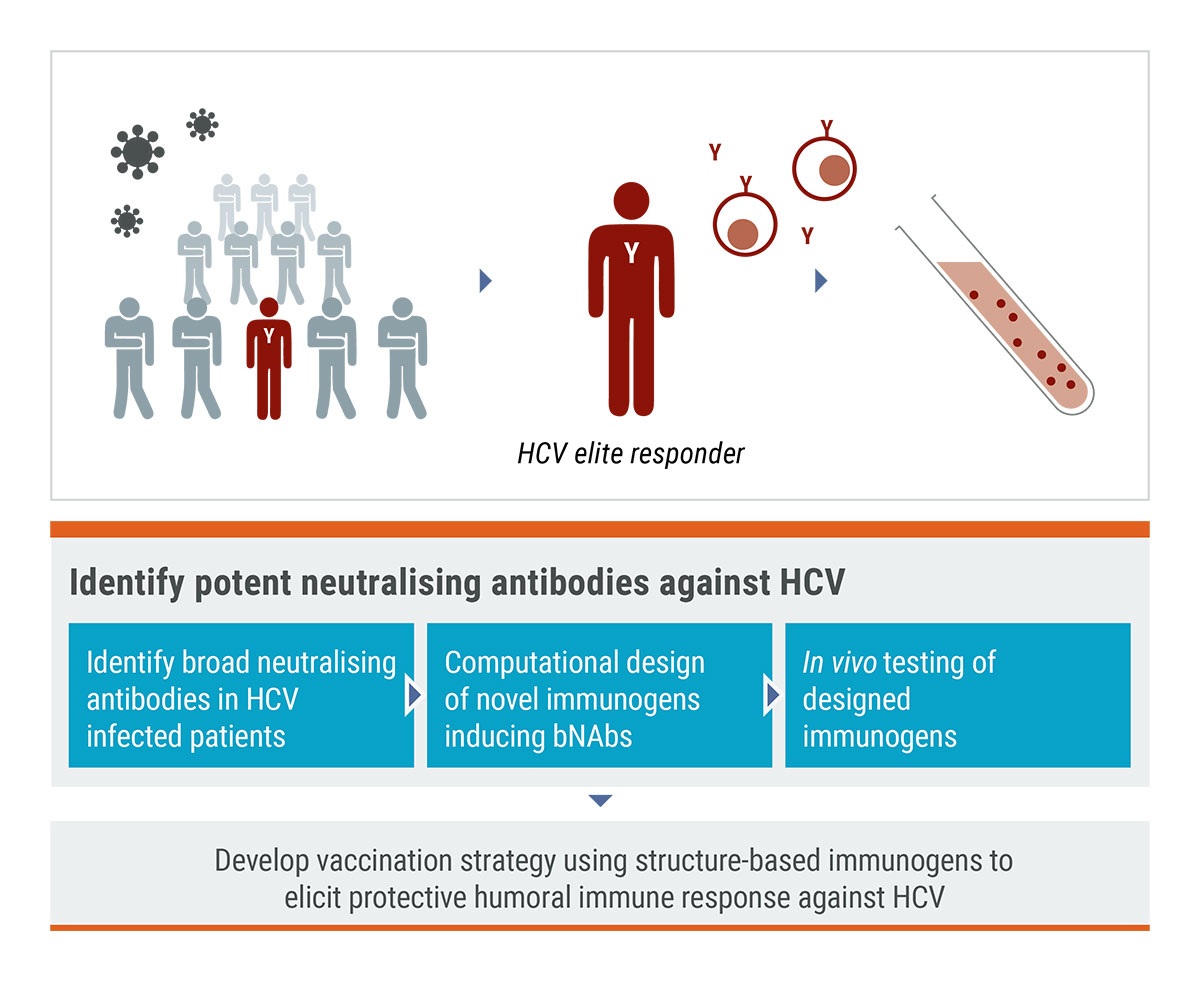
Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization. Weber T, Potthoff J, Bizu S, Labuhn M, Dold L, Schoofs T, Horning M, Ercanoglu MS, Kreer C, Gieselmann L, Vanshylla K, Langhans B, Janicki H, Ströh LJ, Knops E, Nierhoff D, Spengler U, Kaiser R, Bjorkman PJ, Krey T, Bankwitz D, Pfeifer N, Pietschmann T, Flyak AI, Klein F. Immunity.
The Human Liver-Expressed Lectin CD302 Restricts Hepatitis C Virus Infection. Reinecke B, Frericks N, Lauber C, Dinkelborg K, Matthaei A, Vondran FWR, Behrendt P, Haid S, Brown RJP, Pietschmann T. J Virol.
T. Liver-expressed Cd302 and Cr1l limit hepatitis C virus cross-species transmission to mice. Brown RJP, Tegtmeyer B, Sheldon J, Khera T, Anggakusuma, Todt D, Vieyres G, Weller R, Joecks S, Zhang Y, Sake S, Bankwitz D, Welsch K, Ginkel C, Engelmann M, Gerold G, Steinmann E, Yuan Q, Ott M, Vondran FWR, Krey T, Ströh LJ, Miskey C, Ivics Z, Herder V, Baumgärtner W, Lauber C, Seifert M, Tarr AW, McClure CP, Randall G, Baktash Y, Ploss A, Thi VLD, Michailidis E, Saeed M, Verhoye L, Meuleman P, Goedecke N, Wirth D, Rice CM, Pietschmann Sci Adv.
Global and local envelope protein dynamics of hepatitis C virus determine broad antibody sensitivity. Augestad EH, Castelli M, Clementi N, Ströh LJ, Krey T, Burioni R, Mancini N, Bukh J, Prentoe J. Sci Adv.
Publications 2021
Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization. Weber T, Potthoff J, Bizu S, Labuhn M, Dold L, Schoofs T, Horning M, Ercanoglu MS, Kreer C, Gieselmann L, Vanshylla K, Langhans B, Janicki H, Ströh LJ, Knops E, Nierhoff D, Spengler U, Kaiser R, Bjorkman PJ, Krey T, Bankwitz D, Pfeifer N, Pietschmann T, Flyak AI, Klein F. Immunity.
EpitopeVec: Linear Epitope Prediction Using Deep Protein Sequence Embeddings. Bioinformatics. Bahai A, Asgari E, Mofrad MRK, Kloetgen A, McHardy AC.
Imprint of unconventional T-cell response in acute hepatitis C persists despite successful early antiviral treatment. Du Y, Khera T, Strunz B, Deterding K, Todt D, Woller N, Engelskircher SA, Hardtke S, Port K, Ponzetta A, Steinmann E, Cornberg M, Hengst J, Björkström NK, Wedemeyer H; HepNet Acute HCV Ⅳ Study Group. Eur J Immunol.
Publications 2020
Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Martens R, Permanyer M, Werth K, Yu K, Braun A, Halle O, Halle S, Patzer GE, Bošnjak B, Kiefer F, Janssen A, Friedrichsen M, Poetzsch J, Kohli K, Lueder Y, Gutierrez Jauregui R, Eckert N, Worbs T, Galla M, Förster R. Nat Commun.
Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralising antibodies. Bankwitz D, Bahai A, Labuhn M, Doepke M, Ginkel C, Khera T, Todt D, Ströh LJ, Dold L, Klein F, Klawonn F, Krey T, Behrendt P, Cornberg M, McHardy AC, Pietschmann T. Gut. 2020
Liver-expressed Cd302 and Cr1l limit hepatitis C virus cross-species transmission to mice. Brown RJP, Tegtmeyer B, Sheldon J, Khera T, Anggakusuma, Todt D, Vieyres G, Weller R, Joecks S, Zhang Y, Sake S, Bankwitz D, Welsch K, Ginkel C, Engelmann M, Gerold G, Steinmann E, Yuan Q, Ott M, Vondran FWR, Krey T, Ströh LJ, Miskey C, Ivics Z, Herder V, Baumgärtner W, Lauber C, Seifert M, Tarr AW, McClure CP, Randall G, Baktash Y, Ploss A, Thi VLD, Michailidis E, Saeed M, Verhoye L, Meuleman P, Goedecke N, Wirth D, Rice CM, Pietschmann Sci Adv.
HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development. Ströh LJ, Krey T. Int J Mol Sci.
Global and local envelope protein dynamics of hepatitis C virus determine broad antibody sensitivity. Augestad EH, Castelli M, Clementi N, Ströh LJ, Krey T, Burioni R, Mancini N, Bukh J, Prentoe J. Sci Adv.
De novo protein design enables the precise induction of RSV-neutralizing antibodies. Sesterhenn F, Yang C, Bonet J, Cramer JT, Wen X, Wang Y, Chiang CI, Abriata LA, Kucharska I, Castoro G, Vollers SS, Galloux M, Dheilly E, Rosset S, Corthésy P, Georgeon S, Villard M, Richard CA, Descamps D, Delgado T, Oricchio E, Rameix-Welti MA, Más V, Ervin S, Eléouët JF, Riffault S, Bates JT, Julien JP, Li Y, Jardetzky T, Krey T, Correia BE. Science.
Efficient acute and chronic infection of stem cell-derived hepatocytes by hepatitis C virus. Carpentier A, Sheldon J, Vondran FWR, Brown RJ, Pietschmann T. Gut.