Which paths lead to new inhibitors against human adenoviruses?
What is this research project about?
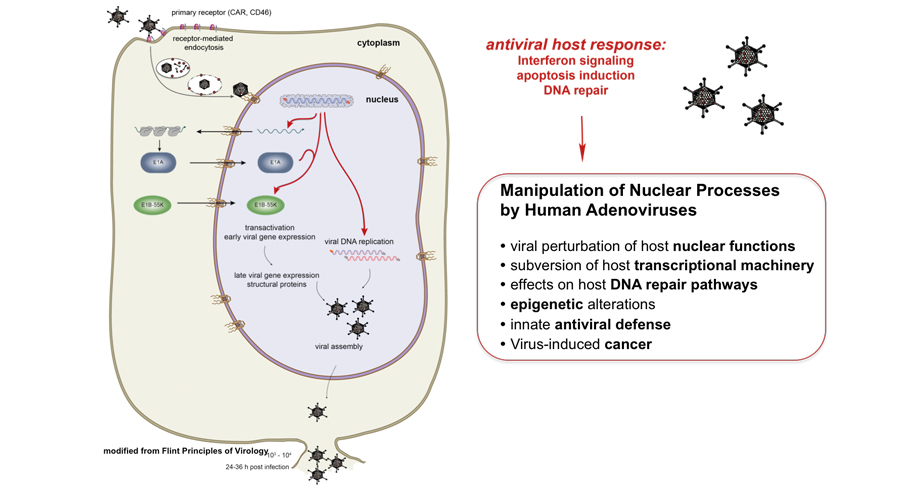
Human adenoviruses (HAdV) are widespread pathogens of the respiratory tract, digestive tract and urinary tract that cause highly infectious follicular epidemic keratoconjunctivitis, i.e. simultaneous inflammation of the conjunctiva and cornea of the eye, and fatal diseases in immunocompromised individuals. Alarmingly, new types have recently been reported that predominantly affect the lungs and can kill healthy people. In the absence of HAdV-specific chemotherapy, a future challenge for basic research will be to provide the background for innovative future antiviral intervention strategies.
HAdV are also commonly used as viral vectors in vaccination strategies (SARS-CoV), gene therapy and tumour therapy because of their high gene delivery efficacy and ease of manipulation. Nevertheless, there are still many gaps in basic knowledge that need to be filled by future discoveries with immediate translation into clinical applications.
What’s the current status?
To date, there is no effective chemotherapeutic drug to treat HAdV infections. Common antiviral treatments such as cidovovir and ribavirin only limit HAdV, but cannot cure severe infections or save patients. Therefore, there is an urgent need to understand the unknown collaborations between virus and host in the first steps of infection in order to develop new antiviral inhibitors that effectively prevent HAdV-mediated disease and mortality.
We are working to identify and characterise missing steps that allow HAdV to introduce genomes into a cell and convert viral chromatin to an active state to promote viral replication or amplify persistent infections with potential and often life-threatening reactivation processes, particularly in immunocompromised individuals, infants and young children.
How do we get there?
We are currently working to identify and characterise the missing steps that allow human pathogenic DNA viruses to introduce their genome into an infected cell, epigenetically transform it into either a transcriptionally active template for early gene expression or a persistence reservoir, and understand the initial host responses, particularly through PML nuclear bodies (PML-NBs), SUMOylation and ubiquitinylation.