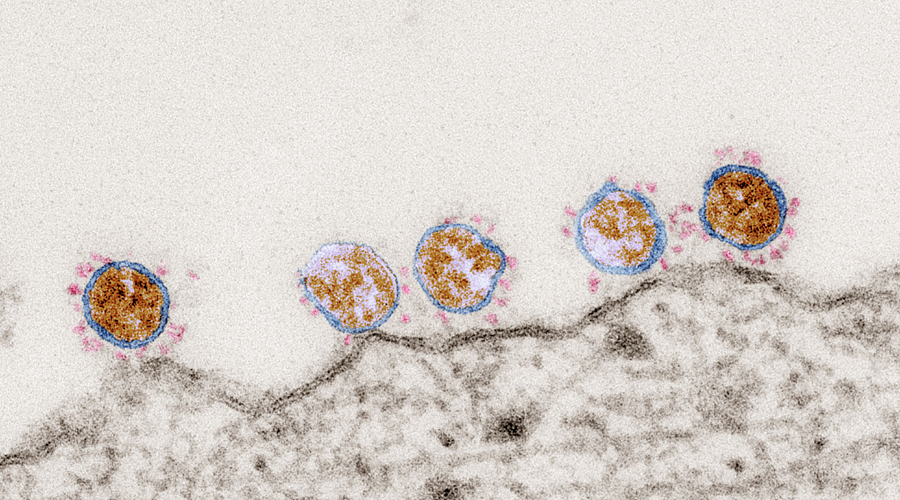
Focus on SARS-CoV-2
SARS-CoV-2 and the clinical features of COVID-19 dramatically highlight the need to better understand why some patients are so strikingly more susceptible to a viral respiratory pathogen. Several researchers involved in RESIST are successfully working to solve this challenge. They are seeking new diagnostic methods, improving vaccines and cloning broadly neutralising monoclonal antibodies.
On this page, we have bundled publications on research related to SARS-CoV-2, as well as news related to the topic of corona infections. It also contains videos and press releases explaining the research, as well as questions and their answers about the topic. We will be happy to keep you up to date.
RESIST Publications on SARS-CoV-2
- Persicamidines-Unprecedented Sesquarterpenoids with Potent Antiviral Bioactivity against Coronaviruses Keller L, Oueis E, Kaur A, Safaei N, Kirsch SH, Gunesch AP, Haid S, Rand U, Čičin-Šain L, Fu C, Wink J, Pietschmann T, Müller R. Angew Chem Int Ed Engl. 2023 Feb 1;62(6):e202214595. doi: 10.1002/anie.202214595. Epub 2023 Jan 4. PMID: 36422061; PMCID: PMC10107436.
- COVID-19 in pediatric lung transplant recipients: Clinical course and outcome. J Heart Lung Transplant. Schütz K, Davids J, Petrik B, Zychlinsky Scharff A, Carlens J, Heim A, Salman J, Ius F, Bobylev D, Hansen G, Müller C, Schwerk N. 2023 Apr;42(4):533-538. doi: 10.1016/j.healun.2022.11.006. Epub 2022 Dec 5. PMID: 36526496; PMCID: PMC9719846.
-
A validated protocol to UV-inactivate SARS-CoV-2 and herpesvirus-infected cells. PLoS One. 2023 May 10;18(5):e0274065. Soh TK, Pfefferle S, Wurr S, von Possel R, Oestereich L, Rieger T, Uetrecht C, Rosenthal M, Bosse JB. PLoS One. 2023 May 10;18(5):e0274065. doi: 10.1371/journal.pone.0274065. PMID: 37163509; PMCID: PMC10171616.
- Omicron infection-associated T- and B-cell immunity in antigen-naive and triple-COVID-19-vaccinated individuals. Barros-Martins J, Hammerschmidt SI, Morillas Ramos G, Cossmann A, Hetzel L, Odak I, Köhler M, Stankov MV, Ritter C, Friedrichsen M, Ravens I, Schimrock A, Ristenpart J, Janssen A, Willenzon S, Bernhardt G, Lichtinghagen R, Bošnjak B, Behrens GMN, Förster R. Front Immunol. 2023 May 5;14:1166589.
- The effect of Toll-like receptor agonists on the immunogenicity of MVA-SARS-2-S vaccine after intranasal administration in mice. Do KTH, Willenzon S, Ristenpart J, Janssen A, Volz A, Sutter G, Förster R, Bošnjak B. Front Cell Infect Microbiol. 2023 Oct 3;13:1259822. doi: 10.3389/fcimb.2023.1259822. PMID: 37854858; PMCID: PMC10580083.
- Memory B cells anticipate SARS-CoV-2 variants through somatic hypermutation. Bruhn M, Obara M, Chiyyeadu A, Costa B, Salam A, Ziegler A, Waltl I, Pavlou A, Bonifacius A, Hoffmann M, Graalmann T, Pöhlmann S, Eiz-Vesper B, Schambach A, Kalinke U. J Infect. 2024 Jan;88(1):57-60.
- Comparing SARS-CoV-2 variants among children and adolescents in Germany: relative risk of COVID-19-related hospitalization, ICU admission and mortality. Jank M, Oechsle AL, Armann J, Behrends U, Berner R, Chao CM, Diffloth N, Doenhardt M, Hansen G, Hufnagel M, Lander F, Liese JG, Muntau AC, Niehues T, von Both U, Verjans E, Weil K, von Kries R, Schroten H. Infection. 2023 Oct;51(5):1357-1367. doi: 10.1007/s15010-023-01996-y. Epub 2023 Feb 14. PMID: 36787015; PMCID: PMC9925936.
- CYP19A1 mediates severe SARS-CoV-2 disease outcome in males. Stanelle-Bertram S, Beck S, Mounogou NK, Schaumburg B, Stoll F, Al Jawazneh A, Schmal Z, Bai T, Zickler M, Beythien G, Becker K, de la Roi M, Heinrich F, Schulz C, Sauter M, Krasemann S, Lange P, Heinemann A, van Riel D, Leijten L, Bauer L, van den Bosch TPP, Lopuhaä B, Busche T, Wibberg D, Schaudien D, Goldmann T, Lüttjohann A, Ruschinski J, Jania H, Müller Z, Pinho Dos Reis V, Krupp-Buzimkic V, Wolff M, Fallerini C, Baldassarri M, Furini S, Norwood K, Käufer C, Schützenmeister N, von Köckritz-Blickwede M, Schroeder M, Jarczak D, Nierhaus A, Welte T, Kluge S, McHardy AC, Sommer F, Kalinowski J, Krauss-Etschmann S, Richter F, von der Thüsen J, Baumgärtner W, Klingel K, Ondruschka B; GEN-COVID Multicenter Study Group; Renieri A, Gabriel G. Cell Rep Med. 2023 Sep 19;4(9):101152. doi: 10.1016/j.xcrm.2023.101152. Epub 2023 Aug 12. PMID: 37572667; PMCID: PMC10518605.
- Genetic Predisposition to Neurological Complications in Patients with COVID-19. Biomolecules. Sahajpal NS, Hastie AR, Schieck M, Mondal AK, Felde M, van der Made CI, Chou JS, Randolph AG, Illig T, Zody MC, Brownstein CA, Beggs AH, Hoischen A, Chaubey A, Kolhe R; COVID19hostgenomesv Consortium. 2023 Jan 9;13(1):133. doi: 10.3390/biom13010133. PMID: 36671517; PMCID: PMC9855758.
- The MEK1/2-inhibitor ATR-002 efficiently blocks SARS-CoV-2 propagation and alleviates pro-inflammatory cytokine/chemokine responses. Schreiber A, Viemann D, Schöning J, Schloer S, Mecate Zambrano A, Brunotte L, Faist A, Schöfbänker M, Hrincius E, Hoffmann H, Hoffmann M, Pöhlmann S, Rescher U, Planz O, Ludwig S. Cell Mol Life Sci. 2022 Jan 10;79(1):65.
- Optical genome mapping identifies rare structural variations as predisposition factors associated with severe COVID-19. Sahajpal NS, Jill Lai CY, Hastie A, Mondal AK, Dehkordi SR, van der Made CI, Fedrigo O, Al-Ajli F, Jalnapurkar S, Byrska-Bishop M, Kanagal-Shamanna R, Levy B, Schieck M, Illig T, Bacanu SA, Chou JS, Randolph AG, Rojiani AM, Zody MC, Brownstein CA, Beggs AH, Bafna V, Jarvis ED, Hoischen A, Chaubey A, Kolhe R; COVID19hostgenomesv consortium. iScience. 2022 Jan 10:103760.
- Wahrnehmung der Corona-Pandemie durch Neurodermitispatienten – Ergebnisse aus dem Neurodermitisregister TREATgermany. Helmert C, Siegels D, Haufe E, Abraham S, Heratizadeh A, Kleinheinz A, Harder I, Schäkel K, Effendy I, Wollenberg A, Sticherling M, Stahl M, Worm M, Schwichtenberg U, Schwarz B, Rossbacher J, Buck PM, Schenck F, Werfel T, Weidinger S, Schmitt J; das TREATgermany Studienteam. J Dtsch Dermatol Ges. 2022 Jan;20(1):45-58.
- Robust induction of neutralizing antibodies against the SARS-CoV-2 Delta variant after homologous Spikevax or heterologous Vaxzevria-Spikevax vaccination. Hammerschmidt SI, Thurm C, Bošnjak B, Bernhardt G, Reinhold A, Willenzon S, Ritter C, Reinhold D, Schraven B, Förster R. Eur J Immunol. 2022 Feb;52(2):356-359.
- Dysregulated PI3K Signaling in B Cells of CVID Patients. Harder I, Münchhalfen M, Andrieux G, Boerries M, Grimbacher B, Eibel H, Maccari ME, Ehl S, Wienands J, Jellusova J, Warnatz K, Keller B. Cells. 2022 Jan 28;11(3):464. doi: 10.3390/cells11030464.
- NK cell dysfunction in severe COVID-19: TGF-β-induced downregulation of integrin beta-2 restricts NK cell cytotoxicity. Barros-Martins J, Förster R, Bošnjak B. Signal Transduct Target Ther. 2022 Jan 31;7(1):32.
- Clinical development and approval of COVID-19 vaccines. Kalinke U, Barouch DH, Rizzi R, Lagkadinou E, Türeci Ö, Pather S, Neels P. Expert Rev Vaccines. 2022 Feb 14.
- Evolution of biofilm-adapted gene expression profiles in lasR-deficient clinical Pseudomonas aeruginosa isolates. Jeske A, Arce-Rodriguez A, Thöming JG, Tomasch J, Häussler S. NPJ Biofilms Microbiomes. 2022 Feb 14;8(1):6.
- Toll-like receptors matter: plasmacytoid dendritic cells in COVID-19. Becker J, Kalinke U. EMBO J. 2022 Apr 26:e111208. doi: 10.15252/embj.2022111208. Epub ahead of print.
- Longitudinal Tracking of Immune Responses in COVID-19 Convalescents Reveals Absence of Neutralization Activity Against Omicron and Staggered Impairment to Other SARS-CoV-2 Variants of Concern. Odak I, Schultze-Florey CR, Hammerschmidt SI, Ritter C, Willenzon S, Friedrichsen M, Ravens I, Sikora R, Bayir LM, Gutierrez Jauregui R, Bernhardt G, Stankov MV, Cossmann A, Hansen G, Krey T, Cornberg M, Koenecke C, Behrens GMN, Bošnjak B, Förster R. Front Immunol. 2022 Mar 14;13:863039. doi: 10.3389/fimmu.2022.863039. PMID: 35359969; PMCID: PMC8964088.
- Clinical development and approval of COVID-19 vaccines. Expert Rev Vaccines. Kalinke U, Barouch DH, Rizzi R, Lagkadinou E, Türeci Ö, Pather S, Neels P. 2022 May;21(5):609-619.
- Longitudinal Tracking of Immune Responses in COVID-19 Convalescents Reveals Absence of Neutralization Activity Against Omicron and Staggered Impairment to Other SARS-CoV-2 Variants of Concern. Odak I, Schultze-Florey CR, Hammerschmidt SI, Ritter C, Willenzon S, Friedrichsen M, Ravens I, Sikora R, Bayir LM, Gutierrez Jauregui R, Bernhardt G, Stankov MV, Cossmann A, Hansen G, Krey T, Cornberg M, Koenecke C, Behrens GMN, Bošnjak B, Förster R. Front Immunol. 2022 Mar 14;13:863039.
- Testing and isolation to prevent overloaded healthcare facilities and reduce death rates in the SARS-CoV-2 pandemic in Italy. Bandyopadhyay A, Schips M, Mitra T, Khailaie S, Binder SC, Meyer-Hermann M. Commun Med (Lond).
- The impact of the first COVID-19 wave on office-based dermatological care in Germany: a focus on diagnosis, therapy and prescription of biologics. Nordhorn I, Weiss D, Werfel T, Zink A, Schielein MC, Traidl S. Eur J Dermatol.
- BNT162b2-boosted immune responses six months after heterologous or homologous ChAdOx1nCoV-19/BNT162b2 vaccination against COVID-19. Behrens GMN, Barros-Martins J, Cossmann A, Ramos GM, Stankov MV, Odak I, Dopfer-Jablonka A, Hetzel L, Köhler M, Patzer G, Binz C, Ritter C, Friedrichsen M, Schultze-Florey C, Ravens I, Willenzon S, Bubke A, Ristenpart J, Janssen A, Ssebyatika G, Krähling V, Bernhardt G, Hoffmann M, Pöhlmann S, Krey T, Bošnjak B, Hammerschmidt SI, Förster R. Nat Commun. 2022 Aug 18.
- Hide and seek with SARS-CoV-2: spike receptor-binding domain-specific memory B cells still recognize Omicron variant. Signal Transduct Target Ther. Odak I, Förster R, Bošnjak B. 2022 Oct 2;7(1):343.
- Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Palacios-Pedrero, M.Á., Jansen, J.M., Blume, C. et al. Nat Aging 2, 896–905 (2022).
- Binding of Glycans to the SARS CoV-2 Spike Protein, an Open Question: NMR Data on Binding Site Localization, Affinity, and Selectivity. Maass T, Ssebyatika G, Brückner M, Breckwoldt L, Krey T, Mallagaray A, Peters T, Frank M, Creutznacher R. Chemistry. 2022 Dec 20;28(71):e202202614.Epub 2022 Oct 26.
- COVID-19 in pediatric lung transplant recipients: Clinical course and outcome. J Heart Lung Transplant. Schütz K, Davids J, Petrik B, Scharff AZ, Carlens J, Heim A, Salman J, Ius F, Bobylev D, Hansen G, Müller C, Schwerk N. 2022 Dec 5
- Altered and allele-specific open chromatin landscape reveals epigenetic and genetic regulators of innate immunity in COVID-19. Zhang B, Zhang Z, Koeken VACM, Kumar S, Aillaud M, Tsay HC, Liu Z, Kraft ARM, Soon CF, Odak I, Bošnjak B, Vlot A; Deutsche COVID-19 OMICS Initiative (DeCOI); Swertz MA, Ohler U, Geffers R, Illig T, Huehn J, Saliba AE, Sander LE, Förster R, Xu CJ, Cornberg M, Schulte LN, Li Y. Cell Genom. 2022 Dec 2
- Association of SARS-CoV-2 Seropositivity With Myalgic Encephalomyelitis and/or Chronic Fatigue Syndrome Among Children and Adolescents in Germany. Sorg AL, Becht S, Jank M, Armann J, von Both U, Hufnagel M, Lander F, Liese JG, Niehues T, Verjans E, Wetzke M, Stojanov S, Behrends U, Drosten C, Schroten H, von Kries R. JAMA Netw Open. 2022 Sep 1;5(9):e2233454. doi: 10.1001/jamanetworkopen.2022.33454. PMID: 36166227; PMCID: PMC9516317.
- Cross-sectional seroprevalence surveys of SARS-CoV-2 antibodies in children in Germany, June 2020 to May 2021. Sorg AL, Bergfeld L, Jank M, Corman V, Semmler I, Goertz A, Beyerlein A, Verjans E, Wagner N, Von Bernuth H, Lander F, Weil K, Hufnagel M, Spiekerkoetter U, Chao CM, Naehrlich L, Muntau AC, Schulze-Sturm U, Hansen G, Wetzke M, Jung AM, Niehues T, Fricke-Otto S, Von Both U, Huebner J, Behrends U, Liese JG, Schwerk C, Drosten C, Von Kries R, Schroten H. Nat Commun. 2022 Jun 6;13(1):3128. doi: 10.1038/s41467-022-30482-6. PMID: 35668073; PMCID: PMC9170697.
- Differential interferon-α subtype induced immune signatures are associated with suppression of SARS-CoV-2 infection. Schuhenn J, Meister TL, Todt D, Bracht T, Schork K, Billaud JN, Elsner C, Heinen N, Karakoese Z, Haid S, Kumar S, Brunotte L, Eisenacher M, Di Y, Lew J, Falzarano D, Chen J, Yuan Z, Pietschmann T, Wiegmann B, Uebner H, Taube C, Le-Trilling VTK, Trilling M, Krawczyk A, Ludwig S, Sitek B, Steinmann E, Dittmer U, Lavender KJ, Sutter K, Pfaender S. Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2111600119. doi: 10.1073/pnas.2111600119. PMID: 35131898; PMCID: PMC8872780.
- Effects of Pre-Existing Mental Conditions on Fatigue and Psychological Symptoms Post-COVID-19. Homann S, Mikuteit M, Niewolik J, Behrens GMN, Stölting A, Müller F, Schröder D, Heinemann S, Müllenmeister C, El-Sayed I, Happle C, Steffens S, Dopfer-Jablonka A. Int J Environ Res Public Health. 2022 Aug 11;19(16):9924. doi: 10.3390/ijerph19169924. PMID: 36011559; PMCID: PMC9408008.
- Longitudinal Tracking of Immune Responses in COVID-19 Convalescents Reveals Absence of Neutralization Activity Against Omicron and Staggered Impairment to Other SARS-CoV-2 Variants of Concern. Odak I, Schultze-Florey CR, Hammerschmidt SI, Ritter C, Willenzon S, Friedrichsen M, Ravens I, Sikora R, Bayir LM, Gutierrez Jauregui R, Bernhardt G, Stankov MV, Cossmann A, Hansen G, Krey T, Cornberg M, Koenecke C, Behrens GMN, Bošnjak B, Förster R. Front Immunol. 2022 Mar 14;13:863039. doi: 10.3389/fimmu.2022.863039. PMID: 35359969; PMCID: PMC8964088.
- SARS-CoV-2 Viral Load in the Pulmonary Compartment of Critically Ill COVID-19 Patients Correlates with Viral Serum Load and Fatal Outcomes. Ynga-Durand M, Maaß H, Milošević M, Krstanović F, Pribanić Matešić M, Jonjić S, Protić A, Brizić I, Šustić A, Čičin-Šain L. Viruses. 2022 Jun 14;14(6):1292. doi: 10.3390/v14061292. PMID: 35746764; PMCID: PMC9228931.
- The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. McLean G, Kamil J, Lee B, Moore P, Schulz TF, Muik A, Sahin U, Türeci Ö, Pather S. mBio. 2022 Apr 26;13(2):e0297921. doi: 10.1128/mbio.02979-21. Epub 2022 Mar 30. PMID: 35352979; PMCID: PMC9040821.
- Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, Dopfer-Jablonka A, Heidemann A, Ritter C, Friedrichsen M, Schultze-Florey C, Ravens I, Willenzon S, Bubke A, Ristenpart J, Janssen A, Ssebyatika G, Bernhardt G, Münch J, Hoffmann M, Pöhlmann S, Krey T, Bošnjak B, Förster R, Behrens GMN. Nat Med. 2021 Jul 14. doi: 10.1038/s41591-021-01449-9. Online ahead of print. PMID: 34262158
- Neutralization of the SARS-CoV-2 Delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Hammerschmidt SI, Bosnjak B, Bernhardt G, Friedrichsen M, Ravens I, Dopfer-Jablonka A, Hoffmann M, Pöhlmann S, Behrens GMN, Förster R. Cell Mol Immunol. 2021 Oct;18(10):2455-2456. doi: 10.1038/s41423-021-00755-z. Epub 2021 Aug 23. PMID: 34426672; PMCID: PMC8381713.
- Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Tscherne A, Schwarz JH, Rohde C, Kupke A, Kalodimou G, Limpinsel L, Okba NMA, Bošnjak B, Sandrock I, Odak I, Halwe S, Sauerhering L, Brosinski K, Liangliang N, Duell E, Jany S, Freudenstein A, Schmidt J, Werner A, Gellhorn Serra M, Klüver M, Guggemos W, Seilmaier M, Wendtner CM, Förster R, Haagmans BL, Becker S, Sutter G, Volz A. Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2026207118. doi: 10.1073/pnas.2026207118. PMID: 34162739 Free PMC article.
- Development of the reproduction number from coronavirus SARS-CoV-2 case data in Germany and implications for political measures. Khailaie S, Mitra T, Bandyopadhyay A, Schips M, Mascheroni P, Vanella P, Lange B, Binder SC, Meyer-Hermann M. BMC Med. 2021 Jan 28;19(1):32. doi: 10.1186/s12916-020-01884-4. PMID: 33504336 Free PMC article.
- Case Report: Convalescent Plasma Therapy Induced Anti-SARS-CoV-2 T Cell Expansion, NK Cell Maturation and Virus Clearance in a B Cell Deficient Patient After CD19 CAR T Cell Therapy. Bošnjak B, Odak I, Ritter C, Stahl K, Graalmann T, Steinbrück L, Blasczyk R, Falk CS, Schulz TF, Wedemeyer HH, Cornberg M, Ganser A, Förster R, Koenecke C. Front Immunol. 2021 Aug 12;12:721738
- Intranasal Delivery of MVA Vector Vaccine Induces Effective Pulmonary Immunity Against SARS-CoV-2 in Rodents. Bošnjak B, Odak I, Barros-Martins J, Sandrock I, Hammerschmidt SI, Permanyer M, Patzer GE, Greorgiev H, Gutierrez Jauregui R, Tscherne A, Schwarz JH, Kalodimou G, Ssebyatika G, Ciurkiewicz M, Willenzon S, Bubke A, Ristenpart J, Ritter C, Tuchel T, Meyer Zu Natrup C, Shin DL, Clever S, Limpinsel L, Baumgärtner W, Krey T, Volz A, Sutter G, Förster R. Front Immunol. 2021 Nov 11;12:772240.
- Robust induction of neutralizing antibodies against the SARS-CoV-2 Delta variant after homologous Spikevax or heterologous Vaxzevria-Spikevax vaccination. Hammerschmidt SI, Thurm C, Bošnjak B, Bernhardt G, Reinhold A, Willenzon S, Ritter C, Reinhold D, Schraven B, Förster R. Eur J Immunol. 2021 Dec 6.
- Long-Lasting Immunity Against SARS-CoV-2: Dream or Reality? Gussarow D, Bonifacius A, Cossmann A, Stankov MV, Mausberg P, Tischer-Zimmermann S, Gödecke N, Kalinke U, Behrens GMN, Blasczyk R, Eiz-Vesper B. Front Med (Lausanne). 2021 Nov 25;8:770381.
- Conservation of the HBV RNA element epsilon in nackednaviruses reveals ancient origin of protein-primed reverse transcription. Beck J, Seitz S, Lauber C, Nassal M. Proceedings of the National Academy of Sciences 2021.
- Discrimination of SARS-CoV-2 Infections From Other Viral Respiratory Infections by Scent Detection Dogs. Ten Hagen NA, Twele F, Meller S, Jendrny P, Schulz C, von Köckritz-Blickwede M, Osterhaus A, Ebbers H, Pink I, Welte T, Manns MP, Illig T, Fathi A, Addo MM, Nitsche A, Puyskens A, Michel J, Krause E, Ehmann R, von Brunn A, Ernst C, Zwirglmaier K, Wölfel R, Nau A, Philipp E, Engels M, Schalke E, Volk HA. Front Med (Lausanne). 2021 Nov 18;8:749588. doi: 10.3389/fmed.2021.749588. PMID: 34869443; PMCID: PMC8636992.
- Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Krämer B, Knoll R, Bonaguro L, ToVinh M, Raabe J, Astaburuaga-García R, Schulte-Schrepping J, Kaiser KM, Rieke GJ, Bischoff J, Monin MB, Hoffmeister C, Schlabe S, De Domenico E, Reusch N, Händler K, Reynolds G, Blüthgen N, Hack G, Finnemann C, Nischalke HD, Strassburg CP, Stephenson E, Su Y, Gardner L, Yuan D, Chen D, Goldman J, Rosenstiel P, Schmidt SV, Latz E, Hrusovsky K, Ball AJ, Johnson JM, Koenig PA, Schmidt FI, Haniffa M, Heath JR, Kümmerer BM, Keitel V, Jensen B, Stubbemann P, Kurth F, Sander LE, Sawitzki B; Deutsche COVID-19 OMICS Initiative (DeCOI); Aschenbrenner AC, Schultze JL, Nattermann J. Immunity. 2021 Nov 9;54(11):2650-2669.e14. doi: 10.1016/j.immuni.2021.09.002. Epub 2021 Sep 4. PMID: 34592166; PMCID: PMC8416549.
- Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Ruhl L, Pink I, Kühne JF, Beushausen K, Keil J, Christoph S, Sauer A, Boblitz L, Schmidt J, David S, Jäck HM, Roth E, Cornberg M, Schulz TF, Welte T, Höper MM, Falk CS. Signal Transduct Target Ther. 2021 Dec 10;6(1):418. doi: 10.1038/s41392-021-00819-6. PMID: 34893580; PMCID: PMC8661333.
- Exploring beyond clinical routine SARS-CoV-2 serology using MultiCoV-Ab to evaluate endemic coronavirus cross-reactivity. Becker M, Strengert M, Junker D, Kaiser PD, Kerrinnes T, Traenkle B, Dinter H, Häring J, Ghozzi S, Zeck A, Weise F, Peter A, Hörber S, Fink S, Ruoff F, Dulovic A, Bakchoul T, Baillot A, Lohse S, Cornberg M, Illig T, Gottlieb J, Smola S, Karch A, Berger K, Rammensee HG, Schenke-Layland K, Nelde A, Märklin M, Heitmann JS, Walz JS, Templin M, Joos TO, Rothbauer U, Krause G, Schneiderhan-Marra N. Nat Commun. 2021 Feb 19;12(1):1152. doi: 10.1038/s41467-021-20973-3. PMID: 33608538; PMCID: PMC7896075.
- Impaired immune response mediated by prostaglandin E2 promotes severe COVID-19 disease. Ricke-Hoch M, Stelling E, Lasswitz L, Gunesch AP, Kasten M, Zapatero-Belinchón FJ, Brogden G, Gerold G, Pietschmann T, Montiel V, Balligand JL, Facciotti F, Hirsch E, Gausepohl T, Elbahesh H, Rimmelzwaan GF, Höfer A, Kühnel MP, Jonigk D, Eigendorf J, Tegtbur U, Mink L, Scherr M, Illig T, Schambach A, Pfeffer TJ, Hilfiker A, Haverich A, Hilfiker-Kleiner D. PLoS One. 2021 Aug 4;16(8):e0255335. doi: 10.1371/journal.pone.0255335. PMID: 34347801; PMCID: PMC8336874.
- Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP). Horn A, Krist L, Lieb W, Montellano FA, Kohls M, Haas K, Gelbrich G, Bolay-Gehrig SJ, Morbach C, Reese JP, Störk S, Fricke J, Zoller T, Schmidt S, Triller P, Kretzler L, Rönnefarth M, Von Kalle C, Willich SN, Kurth F, Steinbeis F, Witzenrath M, Bahmer T, Hermes A, Krawczak M, Reinke L, Maetzler C, Franzenburg J, Enderle J, Flinspach A, Vehreschild J, Schons M, Illig T, Anton G, Ungethüm K, Finkenberg BC, Gehrig MT, Savaskan N, Heuschmann PU, Keil T, Schreiber S. Infection. 2021 Dec;49(6):1277-1287. doi: 10.1007/s15010-021-01707-5. Epub 2021 Oct 12. PMID: 34642875; PMCID: PMC8508400.
- Mapping the human genetic architecture of COVID-19. COVID-19 Host Genetics Initiative. Nature. 2021 Dec;600(7889):472-477. doi: 10.1038/s41586-021-03767-x. Epub 2021 Jul 8. PMID: 34237774; PMCID: PMC8674144.
- Neurological symptoms and complications in predominantly hospitalized COVID-19 patients: Results of the European multinational Lean European Open Survey on SARS-Infected Patients (LEOSS). Kleineberg NN, Knauss S, Gülke E, Pinnschmidt HO, Jakob CEM, Lingor P, Hellwig K, Berthele A, Höglinger G, Fink GR, Endres M, Gerloff C, Klein C, Stecher M, Classen AY, Rieg S, Borgmann S, Hanses F, Rüthrich MM, Hower M, Tometten L, Haselberger M, Piepel C, Merle U, Dolff S, Degenhardt C, Jensen BO, Vehreschild MJGT, Erber J, Franke C, Warnke C; LEOSS Study Group. Eur J Neurol. 2021 Dec;28(12):3925-3937. doi: 10.1111/ene.15072. Epub 2021 Sep 3. PMID: 34411383; PMCID: PMC8444823.
- Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag F, Weißenborn K, Höglinger G, Maasoumy B, Wedemeyer H, Ganser A. Blood. 2021 Jul 29;138(4):350-353. doi: 10.1182/blood.2021011958. PMID: 34323939; PMCID: PMC8084604.
- Swarm Learning for decentralized and confidential clinical machine learning. Warnat-Herresthal S, Schultze H, Shastry KL, Manamohan S, Mukherjee S, Garg V, Sarveswara R, Händler K, Pickkers P, Aziz NA, Ktena S, Tran F, Bitzer M, Ossowski S, Casadei N, Herr C, Petersheim D, Behrends U, Kern F, Fehlmann T, Schommers P, Lehmann C, Augustin M, Rybniker J, Altmüller J, Mishra N, Bernardes JP, Krämer B, Bonaguro L, Schulte-Schrepping J, De Domenico E, Siever C, Kraut M, Desai M, Monnet B, Saridaki M, Siegel CM, Drews A, Nuesch-Germano M, Theis H, Heyckendorf J, Schreiber S, Kim-Hellmuth S; COVID-19 Aachen Study (COVAS); Nattermann J, Skowasch D, Kurth I, Keller A, Bals R, Nürnberg P, Rieß O, Rosenstiel P, Netea MG, Theis F, Mukherjee S, Backes M, Aschenbrenner AC, Ulas T; Deutsche COVID-19 Omics Initiative (DeCOI); Breteler MMB, Giamarellos-Bourboulis EJ, Kox M, Becker M, Cheran S, Woodacre MS, Goh EL, Schultze JL. Nature. 2021 Jun;594(7862):265-270. doi: 10.1038/s41586-021-03583-3. Epub 2021 May 26. PMID: 34040261; PMCID: PMC8189907.
- The Upper Respiratory Tract of Felids Is Highly Susceptible to SARS-CoV-2 Infection. Krüger N, Rocha C, Runft S, Krüger J, Färber I, Armando F, Leitzen E, Brogden G, Gerold G, Pöhlmann S, Hoffmann M, Baumgärtner W. Int J Mol Sci. 2021 Sep 30;22(19):10636. doi: 10.3390/ijms221910636. PMID: 34638978; PMCID: PMC8508926.
- Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. O’Toole Á, Hill V, Pybus OG, Watts A, Bogoch II, Khan K, Messina JP; COVID-19 Genomics UK (COG-UK) consortium; Network for Genomic Surveillance in South Africa (NGS-SA); Brazil-UK CADDE Genomic Network; Tegally H, Lessells RR, Giandhari J, Pillay S, Tumedi KA, Nyepetsi G, Kebabonye M, Matsheka M, Mine M, Tokajian S, Hassan H, Salloum T, Merhi G, Koweyes J, Geoghegan JL, de Ligt J, Ren X, Storey M, Freed NE, Pattabiraman C, Prasad P, Desai AS, Vasanthapuram R, Schulz TF, Steinbrück L, Stadler T; Swiss Viollier Sequencing Consortium; Parisi A, Bianco A, García de Viedma D, Buenestado-Serrano S, Borges V, Isidro J, Duarte S, Gomes JP, Zuckerman NS, Mandelboim M, Mor O, Seemann T, Arnott A, Draper J, Gall M, Rawlinson W, Deveson I, Schlebusch S, McMahon J, Leong L, Lim CK, Chironna M, Loconsole D, Bal A, Josset L, Holmes E, St George K, Lasek-Nesselquist E, Sikkema RS, Oude Munnink B, Koopmans M, Brytting M, Sudha Rani V, Pavani S, Smura T, Heim A, Kurkela S, Umair M, Salman M, Bartolini B, Rueca M, Drosten C, Wolff T, Silander O, Eggink D, Reusken C, Vennema H, Park A, Carrington C, Sahadeo N, Carr M, Gonzalez G; SEARCH Alliance San Diego; National Virus Reference Laboratory; SeqCOVID-Spain; Danish Covid-19 Genome Consortium (DCGC); Communicable Diseases Genomic Network (CDGN); Dutch National SARS-CoV-2 surveillance program; Division of Emerging Infectious Diseases (KDCA); de Oliveira T, Faria N, Rambaut A, Kraemer MUG. Wellcome Open Res. 2021 Sep 17;6:121. doi: 10.12688/wellcomeopenres.16661.2. PMID: 34095513; PMCID: PMC8176267.
- Safety and efficacy of abatacept in patients with treatment-resistant SARCoidosis (ABASARC) – protocol for a multi-center, single-arm phase IIa trial. Frye BC, Rump IC, Uhlmann A, Schubach F, Ihorst G, Grimbacher B, Zissel G, Quernheim JM.Contemp Clin Trials Commun. 2020 May 29;19:100575. doi: 10.1016/j.conctc.2020.100575. PMID: 32551397; PMCID: PMC7292904.
- Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine. 2020 Jul;57:102885. doi: 10.1016/j.ebiom.2020.102885. Epub 2020 Jul 7. PMID: 32650275; PMCID: PMC7338277. Odak I, Barros-Martins J, Bošnjak B, Stahl K, David S, Wiesner O, Busch M, Hoeper MM, Pink I, Welte T, Cornberg M, Stoll M, Goudeva L, Blasczyk R, Ganser A, Prinz I, Förster R, Koenecke C, Schultze-Florey CR.
- Combating COVID-19: MVA Vector Vaccines Applied to the Respiratory Tract as Promising Approach Toward Protective Immunity in the Lung. Förster R, Fleige H, Sutter G. Front Immunol. 2020 Aug 7;11:1959. doi: 10.3389/fimmu.2020.01959. PMID: 32849655; PMCID: PMC7426738.
- Strategic Anti-SARS-CoV-2 Serology Testing in a Low Prevalence Setting: The COVID-19 Contact (CoCo) Study in Healthcare Professionals. Behrens GMN, Cossmann A, Stankov MV, Schulte B, Streeck H, Förster R, Bosnjak B, Willenzon S, Boeck AL, Thu Tran A, Thiele T, Graalmann T, Kayser MZ, Zychlinsky Scharff A, Dopfer C, Horke A, Pink I, Witte T, Wetzke M, Ernst D, Jablonka A, Happle C. Infect Dis Ther. 2020 Dec;9(4):837-849. doi: 10.1007/s40121-020-00334-1. Epub 2020 Sep 4. PMID: 32886335; PMCID: PMC7472691.
- COVID-19 related reduction in pediatric emergency healthcare utilization – a concerning trend. Dopfer C, Wetzke M, Zychlinsky Scharff A, Mueller F, Dressler F, Baumann U, Sasse M, Hansen G, Jablonka A, Happle C. BMC Pediatr. 2020 Sep 7;20(1):427. doi: 10.1186/s12887-020-02303-6. PMID: 32894080; PMCID: PMC7475725.
- Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Bošnjak B, Stein SC, Willenzon S, Cordes AK, Puppe W, Bernhardt G, Ravens I, Ritter C, Schultze-Florey CR, Gödecke N, Martens J, Kleine-Weber H, Hoffmann M, Cossmann A, Yilmaz M, Pink I, Hoeper MM, Behrens GMN, Pöhlmann S, Blasczyk R, Schulz TF, Förster R. Cell Mol Immunol. 2020 Nov 2:1–9. doi: 10.1038/s41423-020-00573-9. Epub ahead of print. PMID: 33139905; PMCID: PMC7604543.
- Double-edge anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods Bosnjak B, Stein SC, Willenzon S, Cordes AK, Puppe W, Bernhardt G, Ravens I, Ritter C, Schultze-Florey CR, Gödecke N, Martens J, Kleine-Weber H, Hoffmann M, Cossmann A, Yilmaz M, Pink I, Hoeper MM, Behrens GMN, Pöhlmann S, Blasczyk R, Schulz TF, Forster R. Cell Mol Immunol 2020
- A call for a global COVID-19 Neuro Research Coalition. Winkler AS, Knauss S, Schmutzhard E, Leonardi M, Padovani A, Abd-Allah F, Charway-Felli A, Emmrich JV, Umapathi T, Satishchandra P, Hoo FK, Dalmau J, Oreja-Guevara C, Ferreira MLB, Pfausler B, Michael BD, Tagliavini F, Höglinger G, Endres M, Klein C, Hemmer B, Carroll W, Sejvar J, Solomon T. Lancet Neurol. 2020 Jun;19(6):482-484. doi: 10.1016/S1474-4422(20)30150-2. Epub 2020 May 26. Erratum in: Lancet Neurol. 2020 Aug;19(8):e7. PMID: 32470416; PMCID: PMC7250549.
- A molecular pore spans the double membrane of the coronavirus replication organelle. Wolff G, Limpens RWAL, Zevenhoven-Dobbe JC, Laugks U, Zheng S, de Jong AWM, Koning RI, Agard DA, Grünewald K, Koster AJ, Snijder EJ, Bárcena M. Science. 2020 Sep 11;369(6509):1395-1398. doi: 10.1126/science.abd3629. Epub 2020 Aug 6. PMID: 32763915; PMCID: PMC7665310.
- Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K, Wegner F. Front Neurol. 2020 Oct 6;11:576153. doi: 10.3389/fneur.2020.576153. PMID: 33123081; PMCID: PMC7573137.