Why do some people recover spontaneously after therapy disruption?
What is this research project about?
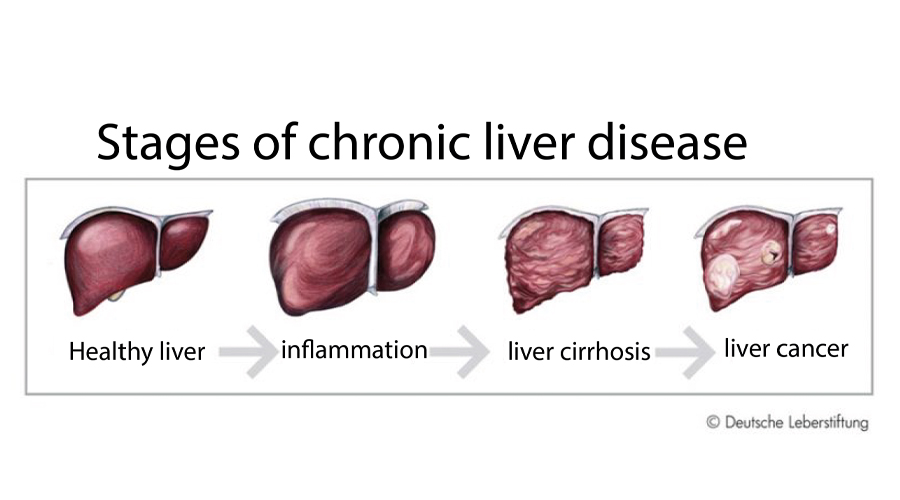
Hepatitis B virus (HBV) is a global clinical challenge. Worldwide more than 250 million people are chronically infected with HBV with 650.000 HBV-related deaths annually. Current treatment options for chronic hepatitis B in industrialized countries are PEG-IFNα or nucleos(t)id-analoga (NA). The therapy with NA can suppress viral replication very efficiently. However, only in a small percentage of patients (less than 1% per year), loss of HBsAg (functional cure of HBV) occurs.
What’s the current status?
NA-treated patients are still at risk of developing liver cirrhosis, liver failure or hepatocellular carcinoma (HCC), since NA therapy only inhibits the generation of new hepatitis B virions, but does not affect the generation of HBsAg and subviral particles. Also, NA therapy does not affect the covalently closed circular HBV DNA (cccDNA), the template of HBV. Therefore, new strategies to induce HBsAg loss are needed.
In previous clinical studies, Cornberg/Kraft proposed a strategy to induce hepatic flares by stopping or interrupting NA therapy. They could show that stop of NA treatment in HBeAg- negative patients is safe and can result in decline of HBsAg levels or even in loss of HBsAg in up to 20 % of the patients. So far, it is still not completely clear, which mechanisms are involved in the increased functional cure in patients with NA treatment interruption. To this end, we have initiated a second prospective study (Terminator 2 study) in order to recruit more patients who will stop long-term NA therapy.
How do we get there?
The project builds on collecting samples from patients enrolled in the the hepatitis B cohort and in the Terminator 2 study. Preliminary results showed that γδ T cell numbers are elevated in HBV patients as compared to healthy controls. So far, nothing is known about the phenotype, TCR repertoire, and antiviral potential of γδ T cells in hepatitis B. While it is known that HBV- specific αβ T cell responses are important for control and clearance of HBV, we hypothesize that γδ T cell responses are also critical for patients which cleared the infection after stop of NA treatment. To monitor T cell responses in the course of infections, we have established NGS technologies that provide comprehensive access to complex antigen receptor repertoires.
This project comprises phenotypical, functional and T cell receptor (TCR) analyses of blood-derived αβ and γδ T cells. Specifically, phenotypical and functional HBV-core specific CD4 and CD8 T cell responses will be analyzed in HLA-typed patients using 16 parameter flow cytometry (e.g. HLA-DRB1 for CD4 and HLA-A2+ for CD8). In parallel we will analyze the TCR repertoire of the dominant CD8 and CD4 T cell responses after FACS-sorting.
To determine and investigate the role of γδ T cells in the Terminator 2 study patient cohort, we use multiplex NGS γδ TCR analyses. In particular, we have set up a pipeline to investigate γδ TCR repertoires by RNA-based multiplex PCR amplicon sequencing and αβ TCR repertoires by RNA-based 5’-RACE PCR amplicon sequencing.
Blood samples from hepatitis patients are scanned.