Which genetic factors lead to severe disease progressions? How does the virus manage to persist in the body permanently and what causes reactivation?
What is this research project about?
Varicella zoster virus (VZV) infects the majority of people in industrialised countries. VZV establishes latency in neurons, remaining lifelong in the infected individual. Reactivation of VZV normally results in shingles, characterized by a painful rash, which may be followed by post-herpetic neuralgia (PHN), the second most common type of neuropathic pain worldwide. PHN is chronic pain that lasts for months after the disappearance of the rash and active VZV replication. Therefore, we hypothesise that VZV imposes epigenetic modifications in key genes involved in pain, leading to long-term changes in expression and activity even once VZV replication and expression has ended. Infection of the central nervous system may lead to encephalitis. Most individuals control VZV infection, whereas in others PHN or life-threatening diseases are more common. The mechanisms leading to latency and reactivation of VZV are unknown. Genetic polymorphisms, mainly affecting immune-related genes, may account for the different outcomes of infection. However, the relevance of most polymorphisms on VZV susceptibility has not been investigated.
What’s the current status?
Drugs to treat VZV disease target the lytic virus without affecting the latent reservoir. A newly developed subunit vaccine protects against zoster and subsequent chronic pain. However, the safety and efficacy of this vaccine has not been tested in most risk groups. Moreover, vaccination is far from universal. The identification of genetic polymorphisms responsible for severe VZV susceptibility and the mechanisms governing latency and reactivation will facilitate the development of drugs to alleviate exacerbated VZV disease.

There is a vaccine that protects against zoster.
How do we get there?
We are a team of clinicians and basic researchers combining a wide range of scientific and technological expertise in the fields of virology, pathogenesis, neurology, dermatology and epigenetics, facilitating the success of the project.
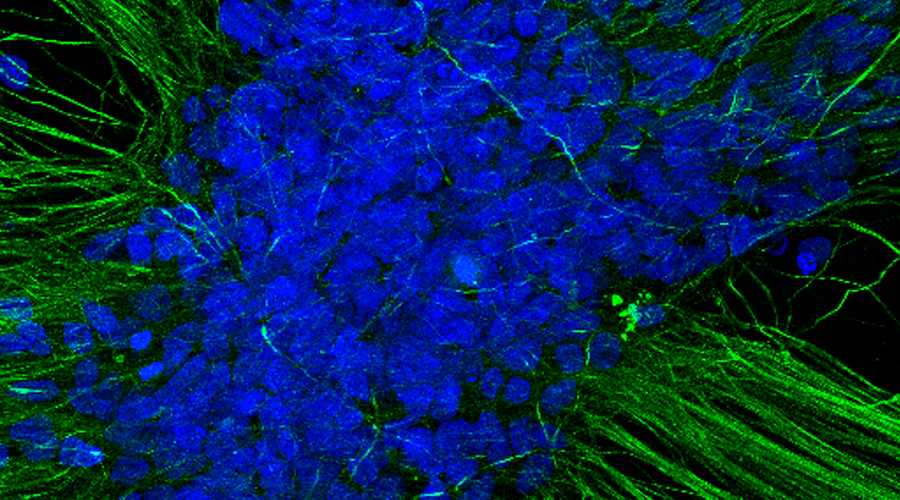
We look into the proposed challenges from the perspective of the virus and the human being. VZV is a human specific virus that establishes latency and reactivates in neurons. Therefore, to investigate VZV and to develop and test hypotheses to inhibit establishment of latency and reactivation, it is necessary to use human neurons. To this end, we have derived human neurons from induced pluripotent stem cells (iPSC) and established VZV latency and reactivation models. With these models we have already started to investigate the process leading to VZV latency.
Our results show that the VZV genome is progressively repressed, inhibiting transcription of its genes. We have also established a collaboration with Trine Mogensen (Aarhus University, Denmark) to determine the role that genetic polymorphisms on RNA polymerase III have on VZV infection, latency and reactivation of human neurons.
For the clinical aims we are recruiting a cohort of patients with VZV reactivation that will be followed clinically and where biomaterials will be ascertained for a biomarker search. Our preliminary data suggest that a metabolomics analysis of the cerebrospinal fluid can predict the complicating involvement of the central nervous system. We will also use data from the HSV cohort. The samples of the RESIST study with the general population of Hannover will serve as a control here.