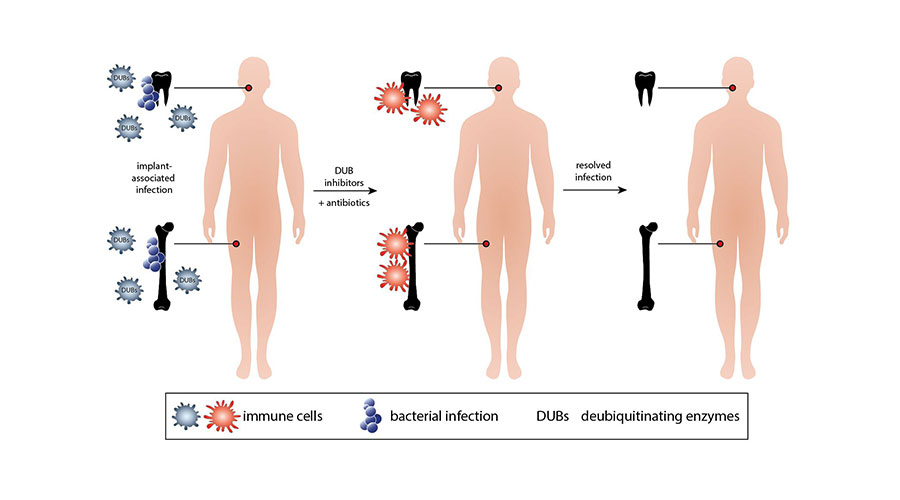
So-called ubiquitins can play an important role during bacterial infections. Are they suitable for new therapeutic approach?
What is this research project about?
The risk and the course of serious bacterial infections are primarily determined by the interaction between the specific bacterium and the infected human. The susceptibility to infection is critically determined by individual host-specific factors including an underlying immunodeficiency, the age and the existence of implants. Bacterial pathogens exploit the impaired resistance of humans to cause infections. At the same time, practically all relevant bacterial pathogens actively weaken the immune system of the infected individuals and thereby exacerbate the infection. Due to the increasing antibiotic resistance of bacteria, antibiotic therapy becomes more challenging. In this regard, the prevention of an impairment of the immune system and its strengthening constitute an innovative therapeutic option.
What’s the current status?
In order to rapidly sense pathogens, the immune system possesses several receptors which identify invading bacteria. Upon engagement of these receptors, defense mechanisms are activated swiftly in immune cells which trigger antibacterial immune reactions within minutes. These immune reactions are decisively dependent on the posttranslational modification of host cell proteins. This includes the ubiquitination of host cell proteins by either the accumulation of ubiquitin molecules on the protein or the cleavage of ubiquitin molecules. In both acute and chronic infections, the ubiquitination status of immunologically important signaling molecules in infected cells and leukocytes is of fundamental importance for the course of infection. For this reason, numerous bacteria have developed mechanisms to manipulate the ubiquitination of host proteins. A therapeutic manipulation of the ubiquitination processes potentially offers new treatment options of infectious diseases beyond antibiotics.
The immune system has to defend itself against pathogens. The photo shows an electron micrograph of the bacterium Acinetobacter baumannii. Source: Gudrun Holland; colouring: Michael Laue/RKI
How do we get there?
In order to evaluate the potential of ubiquitin modulating substances as antiinfectives, we will continue our work on the functional significance and individual domains of deubiquitinating enzymes (DUBs). We have already performed corresponding investigations on the DUBs TNFAIP3, CYLD and OTUB1 and we identified specific domains of these DUBs, which mediate the successful control of bacterial infections (Just et al., 2016; Wex et al., 2016; Wang et al., 2013; Wang et al., 2019; Nishanth et al., 2013). To further study structure/function relation of DUBs, we currently generate new innovative mouse models. In these experiments, we will use established experimental infection models such as listeriosis and additional new models of Staphylococcus aureus-infected implants. Additionally, we will extend these studies to ubiquitin-conjugating enzymes such as the E3-ligase SOCS2. In parallel, we will characterize the expression profile of the 100 known DUBs in samples of human implant-associated infections (cooperation with Prof. Stiesch) and in patients with cholangitis (PD Dr. Heidrich). On the basis of these data, we will develop DUB inhibitors directed against the identified immunologically important domains of DUBs and evaluate these inhibitors in the aforementioned model infections.

Electron microscope image of Listeria monocytogenes. Scale = 1 µm © Petra Kaiser/RKI