What works against SARS-CoV-2? Who falls ill despite vaccination? What role do genes play in the immune response? Numerous questions continue to revolve around the topic of corona. In order to be able to answer them, the “COVID-19 Research Network Lower Saxony” (COFONI) is funding 13 projects with a total of about six million euros. Six of these projects are led by scientists who also conduct research in RESIST or are associated with RESIST. The projects are each supported with up to 500,000 euros and could start between February and April 2022:
The best test
To be able to inhibit SARS-CoV-2 infections with antibodies – that is the goal of the project “Activity of human broadly neutralising antibodies in a SARS-CoV-2 primate model”, which is headed by Prof. Schulz and whose co-applicant is also RESIST researcher Prof. Krey. The researcher’s aim is to ensure that the antibodies can neutralise as many SARS-CoV-2 variants as possible – and if possible also closely related animal coronaviruses that infect human cells and could therefore possibly be transmitted to humans in the future and cause further outbreaks.
Researchers have already succeeded in developing so-called broadly neutralising antibodies. These can specifically inhibit the original SARS-CoV-2 virus, all variants that have appeared so far and even related beta-corona viruses – for example, viruses from pangolins and bats that are related to SARS-CoV-2. The team has also already demonstrated that several of these broadly neutralising antibodies can protect hamsters from infection with both the original and Omicron variants of SARS-CoV-2. “Now we want to test the best of these antibodies in a non-human primate model and develop new antibodies with an even broader neutralising potential,” says Prof Schulz. Prof. Schulz and Prof. Krey are conducting the project with Prof. Pöhlmann (German Primate Centre, DPZ).
Preventing escape
The project “Preclinical development of a monoclonal antibody against SARS-CoV-2 (PREHUMAB)”, coordinated by Prof. Kalinke, also revolves around highly potent SARS-CoV-2 neutralising monoclonal antibodies. His team has already succeeded in developing highly potent SARS-CoV-2 neutralising monoclonal antibodies from memory B cells of recovered COVID-19 patients. “We will now further develop these as so-called multivalent bispecific reagents. The aim is to use these reagents to prevent the emergence of variants of the virus that can escape the immune defences,” says Prof Kalinke.
In addition, the team will test different ways to bring such reagents to expression in the lung epithelium. In addition to experiments in mice and hamsters, preclinical investigations in non-human primates are also planned. Thus, the prerequisites for a clinical phase I investigation in subjects of the most promising antibodies will be fulfilled. Prof. Kalinke is conducting the project together with Prof. Schambach (MHH) and Prof. Pöhlmann (DPZ).
New binding sites sought
Evasive variants of the virus are also the topic of the project “Prediction of Immune Evasion Mutants (PREMUS)” coordinated by Prof. Čičin-Šain. The SARS-CoV-2 coronavirus needs the spike proteins on its surface to bind to specific receptors on the surface of human cells and thus be able to invade the cells. The corresponding binding site of the spike protein is called the receptor binding domain (RBD). Antibodies of the human immune system can bind to the RBD and thus ensure that the viruses cannot penetrate the cells.
“PREMUS aims to identify mutations in this binding site of the spike protein that can prevent currently available monoclonal antibodies from doing anything against the viruses,” says Prof. Čičin-Šain. The research team plans to create a library of mutations of these RBDs. The long-term goal is to identify binding sites of the virus for antibodies, but which are not able to mutate. This should prevent the viruses from using mutations to prevent the antibodies from binding and thus escaping the immune system. The project’s applicants are Prof. Čičin-Šain, Prof. Dübel and Prof. Hust (TU Braunschweig) and Prof. Pöhlmann (DPZ).
The response of the ageing immune system
The project “LISE – Long-term Immune Response of Older Individuals against SARS-CoV-2”, coordinated by Prof. Hühn, revolves around the long-term consequences of a SARS-CoV-2 infection in older people. The team is looking at how strong the cellular and humoral immune response is in older people after SARS-CoV-2 vaccination, how long it lasts and how it declines over time. The applicants also include Prof. Förster, Prof. Li, Prof. Illig, Prof. Pöhlmann (DPZ), Dr. Rösner and Prof. Werfel.
The researchers are also investigating whether there are different patterns of immune response and which molecular mechanisms underlie them. “In addition, we are addressing the extent to which vaccination has induced antibodies that are able to neutralise current and future variants,” says Prof. Hühn. Further questions are: Does the cellular composition of the immune system correlate with the ability to mount a protective immune response after vaccination? Which fully vaccinated individuals are at high risk of infection with current and also future virus variants?
The Lower Saxony Ministry of Science and Culture provides special funding for LISE, as it is a project to research the long-term effects of COVID-19.
Prof. Hühn’s team applies multi-omics analyses, integrates the SARS-CoV-2 specific data with existing multi-omics data and also considers personal risk factors. Multi-omics is an analysis approach in which the data sets are multiple “ome”. For example, the genome, all material carriers of a cell’s heritable information; the transcriptome, all gene transcripts present in a cell; or the epigenome, all chemical modifications of DNA and histone proteins that temporarily determine the activity of genes and thus the functional properties of the cell.
Prediction through multi-omics data
The project coordinated by Prof. Yang Li, “Revealing genetic regulation of immune response to SARS-CoV2 infection using single-cell omics approaches,” also uses multi-omics analysis. Since the extent of disease varies widely among individuals, she addresses the question of what role genetic regulation plays in the immune system’s response to SARS-CoV-2 infection. Prof. Cornberg is also the project’s co-applicant.
The team is studying mechanisms of immune response and disease progression at the single-cell level, including using the genome, transcriptome and epigenome of patients. “We have already been able to show in previous studies that the human immune response to infections is determined by genetic factors, among others, and can be predicted by integrating multi-omics data,” says Prof. Li. “Therefore, we expect that we can also use ‘omics data’ to identify the molecular changes in COVID-19 patients and make immune system responses to SARS-CoV-2 infections.” The results should help make predictions about disease progression and develop effective therapies.
The target: proteases
Prof. Čičin-Šain wants to find out what role a very specific protein plays in SARS-CoV-2 infection in the PROTECT project he is coordinating: “The role of TMPRSS2 proteolysis in the spread of SARS-CoV-2 in cells of the respiratory tract and in vivo”. In addition to himself, the applicants are Prof. Pöhlmann (DPZ), Prof. Ulrich (MHH) and Prof. Braun (Fraunhofer ITEM). Together with his team, he is concentrating on the transmembrane serine protease 2 (TMPRSS2). This is a cell surface protein that occurs in the respiratory tract and is involved in the cleavage of peptide bonds of certain proteins. The central question here is: What role does this protein play in the entry of the SARS-CoV-2 virus into the cell as well as in the infection?
The team assumes that this cellular protease could be a molecular target for therapeutic strategies against COVID-19 disease. “This is particularly interesting since the emergence of the Omicron variant, which has shown in vitro that the TMPRSS2 protease is less clearly required,” says Prof. Čičin-Šain. The laboratory teams involved in this project will study SARS-CoV-2 infection in human tissue cultures as well as in lung sections and in rodent and primate models.
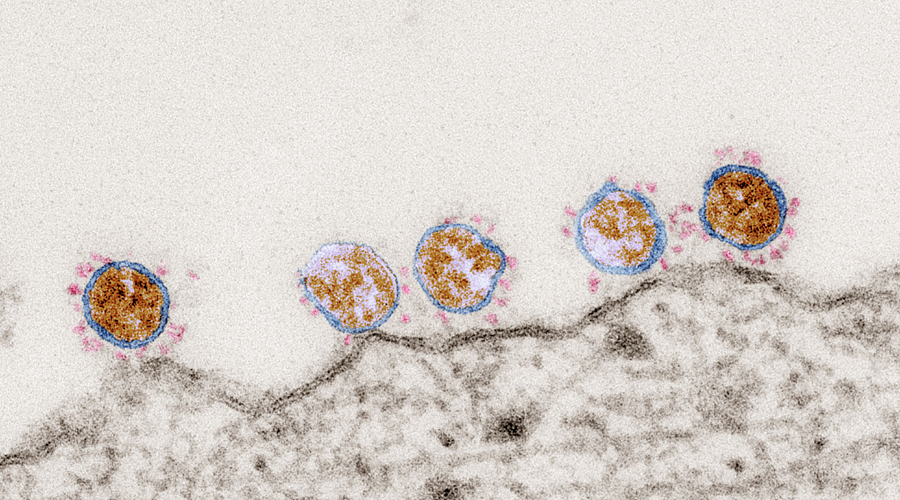
The figure shows SARS coronavirus 2 particles on the surface of a cell (electron micrograph, ultra-thin section)
Copyright: Tobias Hoffmann, Robert Koch-Institut (RKI), 2020